Propofol-BFS – A Breakthrough in Anesthesia from CPC1HN
Propofol is the preferred intravenous anesthetic for induction and maintenance of general anesthesia, used globally due to its rapid onset, predictable pharmacokinetics, and short recovery time. It is widely applied in both adults and children over 3 years of age.
Propofol-BFS – A Breakthrough in Anesthesia from CPC1HN
1. Propofol – The Leading Choice for Anesthesia Induction
Propofol is the preferred intravenous anesthetic for induction and maintenance of general anesthesia, used globally due to its rapid onset, predictable pharmacokinetics, and short recovery time. It is widely applied in both adults and children over 3 years of age.
See more at: Sneyd JR et al. The International Consensus Guideline on Propofol Use. Br J Anaesth, 2020. DOI: 10.1016/j.bja.2020.02.023
2. Ultra-Fine Emulsion for Injection Comfort
Unlike other normal propofol formulations that often contain coarse lipid droplets (which can leak free oil into the bloodstream and cause injection pain or lipid embolism), Propofol-BFS by CPC1HN features:
-
Uniform, micro-dispersed emulsion
-
MCT/LCT oil phase combination → helps reduce free propofol concentration
-
Reduced vascular irritation during injection
-
Minimal risk of late-onset injection pain, commonly reported with other emulsions
Clinical data confirm that a lower free propofol fraction correlates with reduced pain and vascular reactivity.
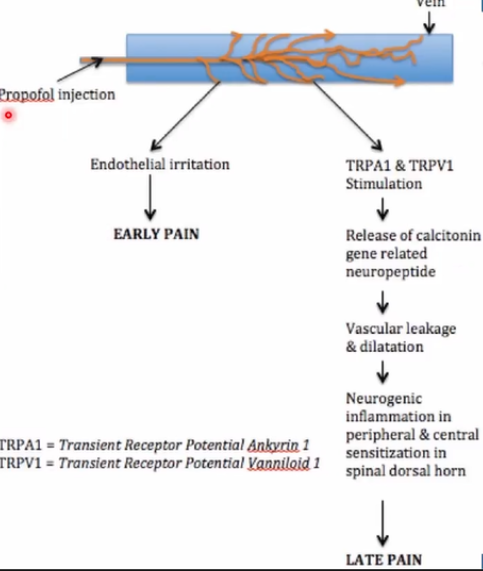 (lấy ảnh trong slide training), tiêu đề: Free propofol concentrations ≥ 50 mcg/mL are associated with a significant increase in the incidence and severity of injection pain.
(lấy ảnh trong slide training), tiêu đề: Free propofol concentrations ≥ 50 mcg/mL are associated with a significant increase in the incidence and severity of injection pain.
See more at: Girdler NM et al. "Pain on injection of propofol: comparison of MCT/LCT and LCT formulations." Anaesthesia, 2000. DOI: 10.1046/j.1365-2044.2000.01533.x
3. Pediatric Safety Profile
Propofol-BFS is approved for use in children ≥3 years old and is frequently used in pediatric procedural sedation and short surgeries. Internal surveillance data from CPC1HN indicate no recorded adverse drug reactions (ADR) to date.
This aligns with published data indicating the favorable safety profile of propofol in pediatric populations when formulated with controlled emulsion profiles.
See more at: Cravero JP et al. The Incidence and Nature of Adverse Events During Pediatric Sedation. Pediatrics, 2006. DOI: 10.1542/peds.2005-1542
4. BFS Technology: 100% Sterility, 0% Contamination Risk
Propofol-BFS is manufactured using Blow-Fill-Seal (BFS) technology — a closed, aseptic system where container formation, filling, and sealing occur in a single step without human contact. The BFS format ensures:
-
No need for preservatives
-
No reconstitution
-
No contamination risk during handling
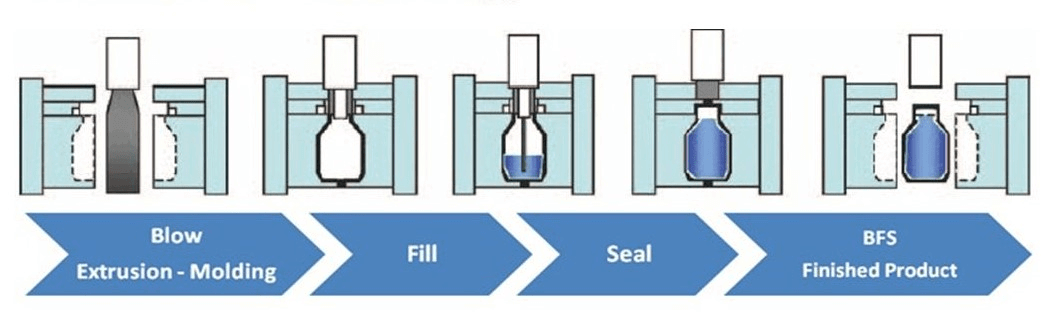
5. Why Propofol-BFS Outperforms Conventional Brands
|
Feature |
Propofol-BFS (CPC1HN) |
Conventional Propofol |
|
Emulsion size |
Ultra-fine, homogenous |
Coarse droplets, unstable |
|
Oil phase |
Balanced MCT/LCT |
LCT-only, higher free fraction |
|
Injection pain profile |
Rare, minimal |
Common, especially delayed |
|
Packaging |
BFS – fully aseptic, sealed |
Manual vial handling |
|
Pediatric indication |
From 3 years |
Often limited or off-label |
|
Use feedback |
Highly positive, pain-free |
Variable, inconsistent |
6. Conclusion
Propofol-BFS by CPC1HN represents a technologically advanced solution in anesthetic care. With its superior emulsion formulation, pediatric-friendly profile, and BFS packaging, it delivers safety, precision, and user convenience in every dose. It’s not just a product — it’s a clinical upgrade.
See more at: https://cpc1hn.com/products/-11/propofol-BFS
